Testing for Vitamin C Copy
Engaging Questions
-
How does one measure the amount of vitamin C in a food source?
-
Which type of juice has the most vitamin C?
-
Does it make a difference in the amount of vitamin C if the juice is fresh, frozen, or canned?
Teacher Goals
-
You will determine the amount of vitamin C in various products.
-
Using your newly gained knowledge about the amounts of Vitamin C in products, you will make better decisions about food products.
Learner Goals
-
One will be able to determine amounts of Vitamin C in products.
-
Demonstrate protocol use to measure quantity of vitamin C in a food source.
Required Resources
-
- Ring stand, burette clamps or utility clamps, and a burette (or a syringe may be used in place of these items)
- 150 ml beaker
- 250 ml beaker or plastic cup
- 10 ml graduated cylinder
- dropping bottle with 3M hydrochloric acid
- iodine solution
- starch solution in 30 ml dropping bottles
- juices
- safety goggles
Optional Resources
-
The Vitamin C Fact Sheet maintained by the Ohio State University Extension Service, provides a concise summary of facts about vitamin C, sources, and content.
-
This pdf link contains instructions students could follow to learn how to titrate acids and bases. It is a basic hands on review of the procedure.
Steps
-
Vitamin C Chart
Obtain a copy of the Vitamin C Chart attached below. Use this hard copy to begin recording your data. Record whether your juice sample is fresh, frozen, or canned.Optionally consider record data directly into attached LoggerPro for analysis later.
-
Iodine Solution
Obtain a 100 ml beaker that contains 50 ml of iodine solution. -
Burette or Syringe
Set up a ring stand. Clamp a burette to the stand. Fill the burette almost to the top with iodine solution and drain a small amount of the solution into a beaker to remove the air bubbles. Adjust the level to read "zero" or slightly below zero. Be sure to record the beginning level. Remember to read the bottom of the curve of fluid. If using a syringe, fill the syringe to 10 ml. Be sure to remove the air bubbles. -
Standard
A standard is a positive test for a known substance and is used to compare with the results of a tested substance.To establish a standard, pour 10 ml of a 0.1% ascorbic acid solution into a clean 250 ml beaker. Add two drops of dilute hydrochloric acid to the beaker. Add two dropperfuls of starch solution to the beaker. Slowly add iodine solution into the beaker, one drop at a time. Keep swirling the liquid as the iodine is added.
Continue adding the iodine solution until ONE drop turns the solution pale blue after you swirl the beaker; the reaction has reached an end point. Record the amount of iodine used. Note: If you added too much iodine, the solution will be a dark blue - start over!
Record the burette reading at the end point. Then subtract the "burette reading at start" from the "burette reading at the end point." Your answer will be iodine solution used.
Repeat this procedure using a 0.05% ascorbic acid solution.
-
Burette Refill
Refill the burette to zero. -
Juice
Pour 10 ml of fruit or vegetable juice into a clean 250 ml beaker. Add two drops of hydrochloric acid and two dropperfuls of starch solution. Repeat the titration procedure that you used for your standard. Record the amounts. Repeat this procedure for the other juices. -
Calculation of Percentage of Ascorbic Acid
With the results found using the 0.1% ascorbic acid standard, calculate the percentage of ascorbic acid in each type of juice tested. Record this percentage on the chart.
Calculation example:
X = % ascorbic acid in test sample.
If it takes 10 ml of iodine to neutralize the ascorbic acid in the standard, and it takes 7 ml of solution in an unknown juice, then: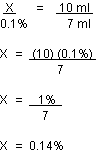
-
Graph and Analyze
If LoggerPro is not available to create and analyze bar graph, use the attached graph paper. Label the horizontal axis with the types of juices tested and the vertical axis with Percent Vitamin C.
Teacher Notes
-
Lesson is appropriate for grades 10, 11, 12.
-
Preparing the Necessary Solutions
- To make two liters of iodine solution, add 1.2 g solid iodine crystals and 6.0 g KI crystals to a 100 ml beaker. Add about 50 ml of water (tap is fine) and stir to dissolve the crystals. Dilute to two liters. Place solutions in small stock bottles or allow students to get their own.
- The starch solution will decompose due to bacteria, so it cannot be kept for long periods. The solution must be used within two to three weeks. Make a paste of 2 g of soluble starch and a small amount of water. Add the mixture slowly to one liter of boiling water and boil for a few minutes until the solution is clear. Place the solution in individual dropping bottles. (If the solution did not clear with boiling, soluble starch was not used. In this case allow the solid to settle; decant and use the clear liquid.)
- A 3 M stock solution of HCl can be prepared by pouring 250 ml of concentrated HCl (approximately 11.7M) into 750 ml of distilled water. Place solutions into small reagent bottles.
-
A graduated cylinder works well to measure the juices. Or use a disposable pipette cut to create a large opening to avoid clogging, can be used.
-
Vitamin C is not a stable compound; heat, light, and exposure to oxygen (air) will cause it to decompose. A good extension would be to measure the decomposition effects heat, light, and air on vitamin C.